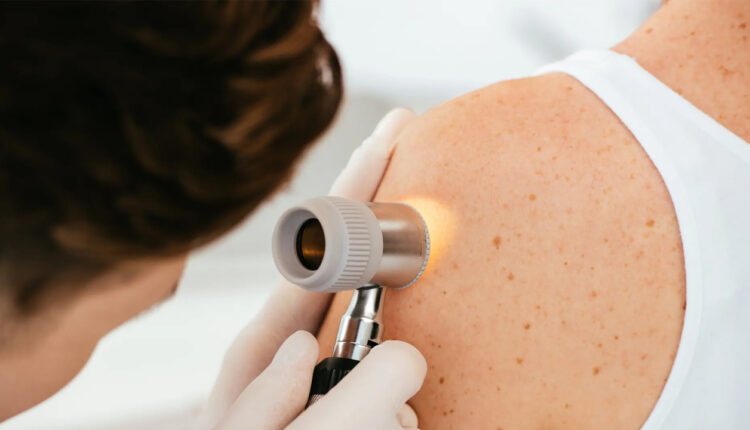
A trial for the world’s first personalised mRNA cancer immunotherapy for melanoma, a type of skin cancer, has begun at University College London Hospital (UCLH), UK.
The work is underpinned by support from NIHR infrastructure.
Melanoma is the most serious form of skin cancer. Immunotherapy is specifically tailored to the genetic signature of each patient’s tumour.
The patient’s immune system recognises and attacks cancerous cells. The aim is to prevent the recurrence of cancer after removal of the tumour.
The treatment is mRNA-based technology developed by Moderna and Merck Sharp and Dohme (MSD).
To personalise the treatment, a sample of the patient’s tumour is removed during surgery.
Then DNA sequencing and artificial intelligence are used to custom-build a therapy that is specific to each tumour.
The Phase 2 study, published in The Lancet, found there was a 49% reduction in the risk of recurrence or death after three years compared with the standard treatment. The new, expanded Phase 3 trial has just been launched.
The new Phase 3 clinical trial is evaluating the combination of mRNA-4157 (V940) and Keytruda (pembrolizumab) versus a current standard of care (pembrolizumab) as a risk-reducing treatment option for patie


ySUuXcxloPhZ
Your article helped me a lot, is there any more related content? Thanks!
824338 651073A really fascinating read, I may possibly nicely not agree entirely, but you do make some quite legitimate factors. 204138
63786 657857fantastic post. Neer knew this, regards for letting me know. 839736
607602 70033The electronic cigarette uses a battery and a small heating component the vaporize the e-liquid. This vapor can then be inhaled and exhaled 820290